Custom Kit Assembly of a Conception Kit
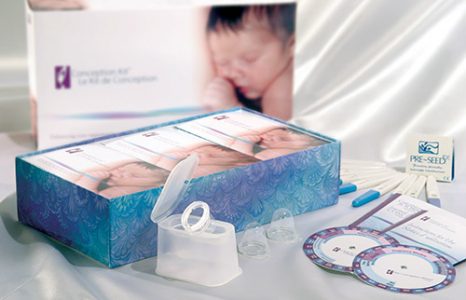
Custom kit assembly solution for mediacal packaging
Liquipak is a full service contract packaging company with capabilities for custom kit assembly, counting, and cartoning. The custom kit assembly project highlighted here involved assembling multiple components into a contraception kit for the medical device industry. The design of the package was co-engineered and featured gloss material which was resistant to spills and stains. Lot codes and expiration dates were also ink-stamped on the flexible packaging material. Each kit was labeled and shrink wrapped, composed of chipboard cartons, packets, various hard parts, and printed materials, the featured dimensions of 10″ in length by 8″ in width.
Quality processes included component and batch traceability, labeling and product accountability, as well as AQL final inspection allowed us to achieve the zero defect requirement. As an FDA registered medical device repackager, Liquipak also follows FDA cGMPs. At Liquipak we operate an ISO 9001:2015 certified facility and adhere to strict internal quality system requirements. To learn more about this project, or the processes used to complete it, see the table below or contact us directly.
Highlights of this Custom Counting and Assembly of a Conception Kit Project
| Product Description | Assembly of multiple components into kits | |
| Capabilities Applied/Processes | Primary: Co-Engineering Packaging Specification Development Assembly |
Secondary: Ink-Stamping – Lot Codes and Expiration Dates Labeling Shrink Wrapping |
| Overall Part Dimensions | Length: 10″ Width: 8″ |
|
| Tightest Tolerances | Zero defects for critical components | |
| Material Finish | Gloss
|
|
| Additional Facts | Components
|
|
| In process testing/inspection performed | Component and batch traceability Labeling and product accountability |
AQL final inspection |
| Industry for Use | Medical – fertility | |
| Volume | 500 + | |
| Delivery/Turnaround Time | 3 weeks | |
| Delivery Location | Alma, Michigan | |
| Standards Met | FDA registered Medical Device Repackager cGMPs Customer’s packaging specifications |
ISO 9001:2015 certification Internal quality system requirements |
| Product Name | Conception Kit |